Objective Ambulatory Tracking
of Activity Levels
The OPOS1 wearable sensor integrates seamlessly with a patient’s existing prosthesis or orthosis to create objective usage and activity level insights for the patient and provider.
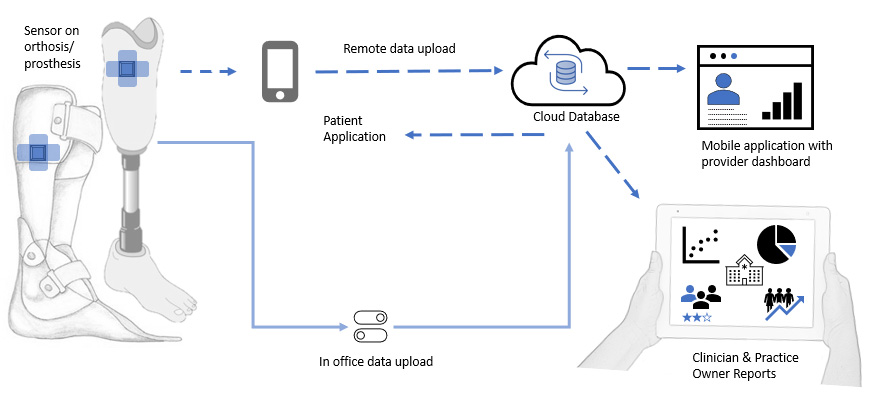
The OPOS1 System

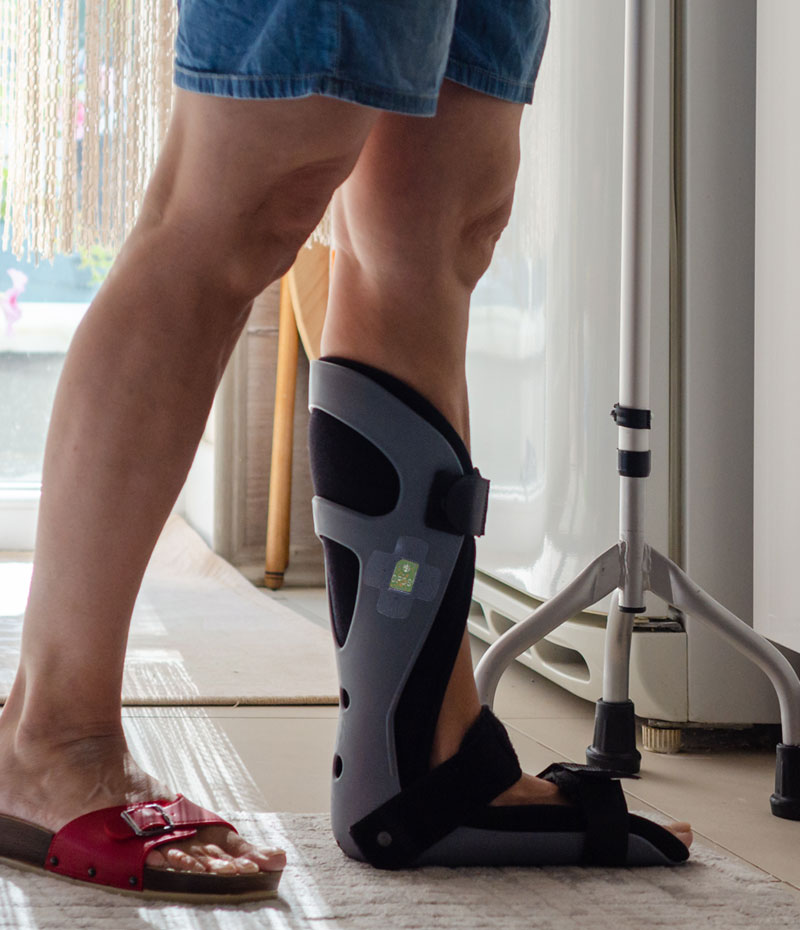
Wearable Sensor
Lightweight, low-profile sensor that is easily mounted directly to the orthosis/prosthesis.
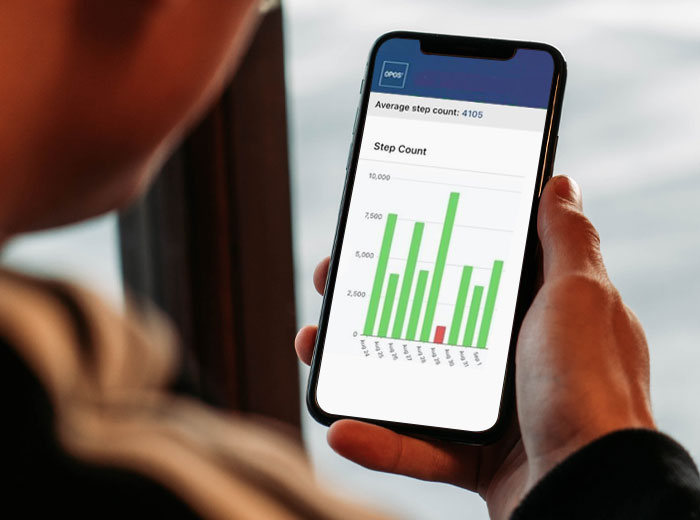
Mobile App
A patient mobile app used for wireless data recording, transmission and visualization.
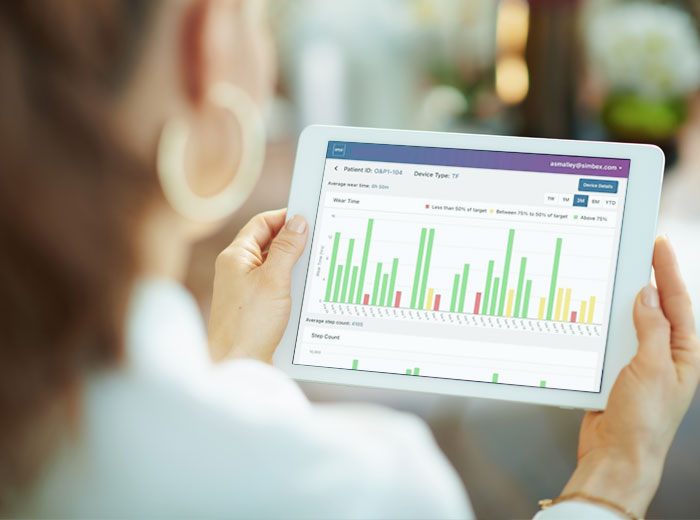
Cloud Database
A cloud platform provides a top-level view of all patients in your practice using the OPOS1 sensor system.
Evidence Based Care, Objective Usage Insights
A comprehensive understanding of how prostheses and orthoses are used in everyday life is essential to the design, fitting, provision, and evaluation of prosthetic and orthotic care.
We provide orthoses and prostheses to help people improve their function and quality of life. This improved function and increased activity level can reduce overall costs for the healthcare system and possibly reduce the effects of chronic disease.
Objective data collected by the OPOS1 system can be combined with provider observations and patient self-reporting to create a cumulative repository of outcomes data on which to base care decisions.
Once patients leave the clinic, providers now have an additional tool providing insight into utilization, which helps increase focus on the abilities and utilization, helping practitioners help patients maintain their functional levels.
There are many reasons why a patient may discontinue or limit the use of an O&P device.
- 01.
Device Factors
Pain and Discomfort
Cosmetic Concerns
Lack of Functional Improvements
Mismatch with Patient Needs or Abilities - 02.
Personal Factors
Depression
Denial of Disability
Negative View of Device
Cognitive Limitations - 03.
Environmental Factors
Accessibility Issues
Social Stigma
Lack of Caregiver Support or Assistance
Patients who do not follow treatment recommendations may experience severe health complications.
“Decreased adherence leads to adverse outcomes that need further medical intervention down the line… increased medical expenditure arises, further burdening our healthcare system” Thatipelli et al (2006)
These issues are more pronounced in vulnerable populations that are at an increased risk of chronic disease progression. The expected mortality rate for a diabetic patient who becomes a below-knee amputee is 66% over a period of 5 years, and this mortality rate increases to 83% over 5 years for diabetic patients with above-knee amputations. One factor thought to contribute to this high mortality rate is the deconditioning that results from reduced mobility, which is exacerbated by an uncomfortable or ill-fitting prosthetic and sequential abandonment.
Objective insight into O&P usage is a crucial first step in comprehending the link between O&P device use and improving health outcomes. Traditional observation of the device’s wear and tear by the provider and surveys or discussions during follow-up visits are unreliable measures, subject to self-reporting, biases, and uncontrolled environmental factors.
With the OPOS1 System, real-world wear-time and activity data can be logged and used to measure clinical outcomes and ultimately improve quality of care.
Ready to Learn More About the OPOS1 System?
Contact us today to learn more about how the OPOS1 system can help deliver better patients' results and your clinical success.
Market Information
OPOS1’s first application is in the O&P and Podiatry market, where more than 185,000 new patients per year are enrolled in the care of over 3,000 O&P facilities providing patient care in the United States.
In addition to traditional O&P practices, orthoses are provided by other healthcare practitioners including podiatrists, physical therapists, and by medical staff in settings such as hospitals, wound care clinics, and orthopedic practices.

O&P
3,200 Clinics

Podiatry
10,500 Practices

Orthopedics
24,00 Groups

Rehabilitation
40,000 Clinics

Research
800 NIH Studies
About the Founder

Dennis Clark, CPO
Founder and president of Clark & Associates, O&P1 and DEC Development with over 50 years of industry experience. Dennis began his career as a staff technician in his father’s business, Dale Clark Prosthetics, during his teenage years. After attending the University of Iowa, he received certifications in Prosthetics and Orthotics at Northwestern University/Fienberg School of Medicine, where he also taught for two years. In 1978, Dennis returned to work for Dale Clark Prosthetics and in 1987, purchased the business. He reorganized the company into Clark & Associates Prosthetics & Orthotics in 2003, where he was President/Partner. From 2003 – 2005, Dennis served as lead prosthetist at Walter Reed Army Medical Center/Prosthetics Department caring for wounded soldiers returning from Iraq and Afghanistan. He has served on the board of directors for the American Academy of Orthotists & Prosthetists (AAOP), president of AAOP-Region 7, and president of The American Board for Certification for Orthotics, Prosthetics and Pedorthics (ABC). Dennis is also an active member of the Amputee Coalition of America (ACA). He has been a frequent lecturer at meetings, conferences, universities and hospitals across the United States. Dennis has been featured in industry, military, and news publications including, O & P Edge, the O & P Almanac, O & P Business News, Popular Mechanics, Biomechanics, Red Orbit, the Waterloo Courier, AR News, Newsmax and The Bayonet. Currently, Dennis is a member of the External Collaborative Panel for the Limb Loss Preservation Registry, a five-year research project sponsored by the National Institute of Health and the Department of Defense.
